It’s a figure folks in the food world hear every day: By the year 2050, the world’s population will double. As agriculture becomes a tougher row to hoe professionally day by day, there’s much concern about how we’ll feed all those extra mouths.

Dr. Zach Lippman
Geneticist Zach Lippman and his team at New York’s famed Cold Spring Harbor Laboratory (home to eight Nobel-winning scientists) might have just handed over a crystal ball of sorts with some recent evolutionary research breakthroughs involving grape and cherry tomatoes that could double yields – and have commercial applications in as little as a year. The technique may apply to other crops as well; Lippman says there are many legitimate candidates.
The same process also results in a jointless plant, preferred by growers for easier harvesting, and lets tomato breeders skip a couple of steps in the traditional breeding sequence to create new jointless varieties.
Even though the process does involve genetic manipulation, it doesn’t involve introducing DNA from external sources. Meaning that the final product is technically not genetically modified and does not have to be identified as GMO.
First, The Egghead Stuff — We’ll Break It Down In A Moment
Interactions among genes – both positive and negative — is called epistasis. Bringing together beneficial traits can surprisingly have negative consequences — sometimes what’s good for the goose makes the gander’s feathers fall out; that’s negative epistasis. But the Lippman team found a way to exploit negative epistasis in a tomato variety to derive untapped yield potential.
Breeding in plants and animals typically involves straightforward addition. As beneficial new traits are discovered — like resistance to drought or larger fruit — they are added to existing prized varieties, delivered via cross-breeding. But every once in a while, adding a beneficial new trait can result in a net subtraction, due to processes deeply hidden within the interactions of genes’ underlying existing and newly added traits.
“Our study provides the first example of which we’re aware of a domestication gene that hindered crop improvement,” says Lippman. “This work illustrates how gene dosage can be exploited to fine-tune and improve major yield traits. It shows that by identifying and dissecting similar cases of negative epistasis in plant and animal breeding, we may be able to break existing productivity barriers in agriculture.”
That’s what Lippman said when his team published the results of their research in a recent issue of the scientific journal Cell.
We asked him to repeat it in simple English and possibly break it down a little — put the fodder down where us mules can get at it — in a recent conversation with SPW.

Cold Spring Harbor Laboratory
“This takes us to the next level because it outlines possibilities that haven’t been really fleshed out yet. But we’re onto something, this is a good first step,” Lippman says. “Is it GMO? It’s a hybrid but it’s more nuanced than that. With the gene editing technology that’s been developed in the last three or four years, you have to go through a genetic engineering step.
“But the product at the end of the day is completely GMO free in the sense that there are no foreign genes or DNA brought in from some other organism. You can regulate the process but not the product. The product itself is non-GMO so why would you regulate it as GMO just because it uses the technology to achieve it?”
Lippman adds that “it is important to subject it to reasonable concerns,” but the bottom line is that thing about the population doubling and all those hungry people.
Virtually all tomatoes carry a natural mutation that happened between 8,000 and 10,000 years ago. That mutation resulted in larger green, leafy caps. Tomato breeders of the time – seriously, even then – intentionally selected and bred varieties that kept that cap. Today we don’t understand why they did it – we just know that trait became part of the DNA in every modern tomato variety.
In the mid-20th century, another natural mutation occurred in a field owned by the Campbell Soup Company. Until that point, all tomatoes had an elbow bend in the joint – the Campbell tomatoes had straight stems – jointless — which meant easier harvesting and storage.
One Plus One Doesn’t Always Equal Two
But the same mutation that led to the larger leafy cap got in the way of breeding new varieties of jointless tomatoes. They often had too many flowering branches – inflorescences. That can increase yield – up to a point, Lippman says. “If tomato or any other plant makes too many flowers, the plant doesn’t have enough resources to turn all those flowers into fruits. The result is actually a decline in fertility.”
Lippman guessed that earlier breeders “missed some important potential in yield” when creating new jointless varieties. To prevent unwanted branching, they bred against that trait.
But Lippman also guessed that if he could better balance the interaction of the genes responsible for branching and flowering, the result would be weak branching – which would mean more flowers and more fruit without overtaxing the plant.
The yield increase comes with jointless easy harvesting and no demonstrable impact on flavor, aroma, shippability and the other traits growers look for.
“In the end you’re going to have to coordinate all of this with traditional breeding to ensure that none of those aspects are compromised. At the moment we don’t see anything dramatic happening to those valuable consumer and production traits. But it has to be done in a very carefully evaluated trials, it has to now be tested in different varieties and different cultivars and see if there’s any effect on some of those traits.
“We focused our efforts mainly on cocktail sized and smaller tomatoes, we realized those were less likely to suffer from negative consequences because it can be a drain on the plant in terms of sugars and acids that are formed as fruits are developing. In larger varieties that may change.”
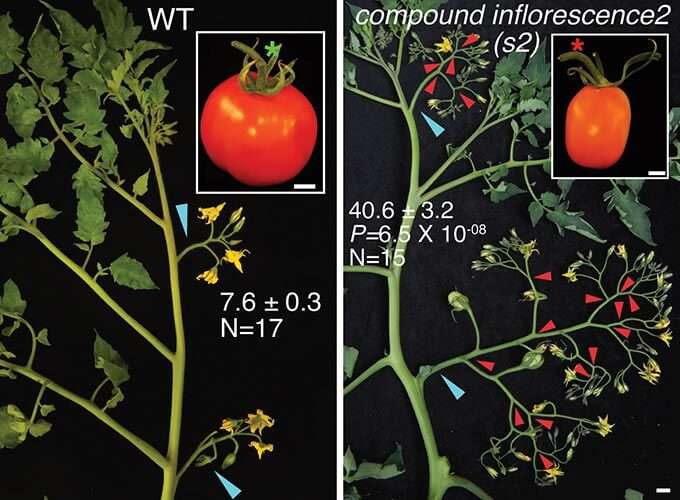
Left: A typical wild tomato plant. Note the simple branches (inflorescences) that lead to several flowers each; and the jointed stem (green asterisk in inset) where the fruit is attached to the branch. Right: mutations in genes that regulate plant architecture result in many more flowers, due to the many branching events marked by red arrowheads. Note in the inset that the stem attaching the fruit to the plant is jointless, a trait desirable to breeders. By tweaking gene dosages, Lippman’s CSHL team has figured out a way to keep jointless plants while inducing weak branching — a sweet spot capturing untapped yield.
Regardless, Lippman says, yields can easily be increased 10-15 percent in larger-fruited plants “and it won’t have an impact.” Further increases may be possible, only more research will tell.
In the end, the result is still more food for the same effort on the same amount of land.
Some breeders are already working with the CSHL research material and one Asian company has committed to development “with open arms”. The “expectation is that field trials will begin soon with some well-known industry names.” Lippman says any breeder with a commercial program could apply the team’s findings and be ready to start commercial trials in roughly a year.
“If they were willing to wake up tomorrow morning and say, ‘We want to do this,’ they could be deploying this knowledge, targeting these genes, within the next year, and it could be in trials very soon after, then put into production. It’s just a matter of them deciding to commit to it and that it’s worth their time and effort.”
Technique May Work With Other Crops, Including Major World Staples
Lippman says it’s sometimes difficult to sell breeders on increased yields because they have more immediately important traits to focus on, the ones consumers are willing to pay for in markets or online today.
Which makes it all the more important that the CSHL research is likely viable for other crops, like potatoes, corn and rice, three sustaining staples that literally feed the world.
“There are a lot of things that will be tomato-specific, but there is a good chunk that will extend to other crops,” Lippman says. “You can customize the process to particular agricultural needs. The way you would do that in tomato would be a specific set of genes, but in rice it might be a different set of genes. But it’s ultimately effecting the same process.”
This is not the first research breakthrough the team has had involving yields.
“There are multiple components to yield,” Lippman says. “We’re now demonstrating over multiple components that you can fine-tune the process that drives those components. So it’s just a matter of building these toolkits, which we’ve shown can be done, and once you have the toolkit you can put in specific tweaks – maybe one set for one variety, one for another. The point is you know when to tweak and how.”



